Highly efficient and cost-effective microfiltration platforms
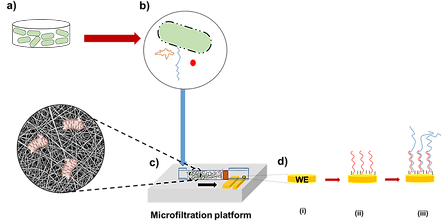
Developing microfluidic chip-based microfiltration platforms for the selective removal, elimination, and filtration of biological target molecules of great interest for various disciplines. The microfiltration platform can be highly effective in removing biological and chemical contaminants, such as bacteria, viruses, dyes, and toxic heavy metal ions. In our novel studies, the suggested platform incorporates nanofiber technology for the filtration of biological target molecules in which an inexpensive, simple, and high-performance filtration capabilities is aimed.
Biomimetic Cancer on a Chip Models for Advancement of Therapy

As a result of rapid urbanization, various important healthcare problems, especially cancer, have dramatically increased. Cancer is an important public health challenge and a leading serious cause of death worldwide. It is accounting for almost 10 million cancer deaths occurred in 2020 which is nearly one in six deaths according to estimates by WHO reports. In this regard, next generation technologies are being developed to solve the problems of complex public health issues by a major transformation to emergence of reliable, low-cost and fast models as an alternative to traditional paradigms. We simulate natural miniature of the target tissues using 3D cancer cells under flow at the microscale for a model to combinatorial drug therapy with toxicity. The environment is formed by mimicking the tumor in vitro environments has more human-specific biochemical factors than the results of experiments in animal models. We also conduct research to elucidate the mechanism of cancer and develop personalized treatment in addition to investigate biotechnological drug candidates through the platform.
Optical-Based pH Sensor for Continuous and Sensitive Measurement for Organ on a Chip

Organ on chip systems are potential new in vitro research tools in the fields of medicine, pharmacology, and biology. In comparison to conventional cell culture platforms, their primary advantage is the enhanced in vivo similarity of the cell culture environment. Monitoring both the cell culture conditions and the biological responses of the cultured cells that is a crucial aspect of these systems. It is very important to ensure the appropriate pH, temperature, humidity and O2 with CO2 levels in order to maintain the microenvironments for the cells in the laboratory. Most cells grow and multiply optimally around pH 7.4. As a result of the decrease in pH from 7 to 6.5, the growth of cells stops and they begin to die between pH 6 and 6.5. The most important issue related to pH measurement in cell, fungal and bacterial culture studies is to be able to measure non-invasively and continuously. Monitoring pH with conventional measurement systems is costly and extremely difficult, especially in small-scale cultivation formats (especially important for repeated laboratory experiments), and invasive measurement techniques can lead to contamination. To address these deficiencies, culture parameters can be established using optical sensors and monitored in a way that does not require an invasive procedure, especially pH. Optical pH sensors can be directly integrated into bioreactors, cell growing vessels (bottles, petri dishes, cylinder bottles, multi-well plates, etc.) and microfluidic chips to ensure non-invasive and continuous pH from the outside. This makes it possible to carry out reliable long-term measurements without the risk of sampling or contamination. Focusing on cutting-edge sensing strategies including those involving organs on chips and other tissue engineering platforms are carried out in our research center.
Personalized 'Organ on a chip' Systems

‘Organ on a chip’ systems are microfluidic 3D human tissue and organ models designed to mimic the important biological and physiological parameters of their in vivo counterparts. They have recently emerged as a platform for personalized medicine and drug screening. Numerous animals die in traditional clinical trials, in addition, the studies last for 10-15 years and the average cost of developing a drug can exceed 2 billion dollars. Therefore, it is expected that these in vitro models will mimic the biomimetic compositions, architectures and functions and will close the large gap between the animal models and the human body. Our work in this field continues with the innovative projects.
Hydrogel Incorporated Microphysiological Tissue-Like Architectures
We study liver cell behavior under fluid flow by co-culturing parenchymal and non-parenchymal cells in parallel microchannels on a chip where in between the two channels there is a pre-injected hydrogel material such as sodium alginate, GelMA and Matrigel that allow interstitial fluid flow. Major architecture of the chip devices we use originally belong to Prof. Roger Kamm’s group from MIT. We investigate the effect of interstitial fluid flow and different types of hydrogel biomaterials on the cell lines for their behaviors co-cultured within the devices where the cells are grown next to a tissue-barrier like architecture fabricated inside a PDMS device.
For more information, click here.
3D Bioprinting in Tissue Engineering

3D bioprinting is a pioneering technology that enables the fabrication of biomimetic, multiscale and multicellular tissues to achieve a highly complex tissue microenvironment, intricate cell architecture, structure-function hierarchy, and tissue-specific composition with mechanical heterogeneity. It is basically a process of integrating living cells with biomaterials that allow the controlled layer-by-layer deposition through hierarchical structural features. Computational studies for the study of tissue growth or tissue fusion after bioprinting can improve the scalability of this technology to produce tissues at desired clinical scales, development of hybrid systems with the integration of different bioprinting modalities, formulation of new bioinks with tunable mechanical and rheological properties. On the other hands, studies 4D bioprinting (stimulus-sensitive) with hydrogels and addressing ethical, social, and regulatory issues have potential futuristic areas that can aid successful clinical translation. In our laboratory, various studies are conducted i.e., organ on a chip, mimicking lung tumor microenvironment, and etc. to investigate and developed novel treatment strategies.
Microfluidic Biosensor for Toxin Detection
The amount of toxin in marine wildlife and even marine birds and mammals has increased due to the increase in pollution in sea water. In this respect, our research group aim is to develop a relatively inexpensive, easy-to-use system that analyzes toxin substances in marine organisms with the microfluidic-biosensor system. Thus, the necessary controls will be made with the detection of target toxins in the frequently consumed seafood and the necessary measures will be taken by monitoring the marine pollution in this sense.
For more information, click here.
Textile Techniques for Tissue Engineering
sad.png)
Chronic wound treatment has recently been of high importance in medical fields. Research shows natural/synthetic polymer structures, hydrogels, foams, electrospinning techniques and their composites can have a potential for use in the field. In particular, natural polymers can be used by forming composites with different materials or structures containing live cells. Polymeric structures, which stand out with their ability to absorb exudate, provide moisture and create barriers, cannot provide a complete solution due to their inability to provide cellular orientation in traditional techniques. Studies include natural polysaccharides like alginate and gelatin methacrylate which can mimics the natural environment. Scaffolds from cell-encapsulated hydrogels are a promising technique for constructing tissues and complex organs. It is worth noting that the encapsulated cells can be protected against the immune system of the patient. On the other hand, one of the main difficulties in the use of cell-encapsulated hydrogels is their low mechanical properties. As a result of this, although it is not possible to process these structures with traditional textile techniques, they are very fragile in terms of their final structure. Therefore, reliable techniques are needed to create strong structures that provide a suitable environment for cell growth. As a result of all this, there are many advantages of developing a textile-based platform from cell-loaded core-shell composite fibers and 3D vascularized 'living' textile scaffolds using weaving, braiding, knitting, winding and embroidery techniques. The core part of the composite fibers used in this structure is obtained from mechanically rigid, biocompatible and biodegradable polymeric fiber, and the soft shell part is obtained from hydrogels seeded with living cells and containing other reagents. This configuration takes advantage of the strength of the core, eliminating the risk of breaking the fiber and facilitating the forming of tissue structures such as weaving, braiding and knitting. For now, we continue our international and national joint studies, especially on muscle tissue and wound dressing composite structures.
For more information, click here
Plant Extract on a Chip

Like all living organisms, plants have their own defense systems by using various molecules. These molecules are bioactive and stable and can have antimicrobial and anticancer properties for human health benefits. Recent advancements in the extraction methods of target molecules make them an intriguing prospect for a novel drug development and disease treatment perspectives. Our research team is working on separating target molecules from the plants extract and investigating their anti-cancer properties. Current techniques for separation and screening of these molecules in the plants are expensive and time-consuming. One of our goals in this study is to design and use a microfluidic chip, to separate target molecules more quickly and cost-efficient manner. Afterward, investigate preliminary anticancer activity in addition to other medicinal properties.
For more information, click here.
Functional High Performance Polymeric Fibers (HiPER)

By an innovative two-step post-processing improvement during the melt-spinning process, we are able to selectively tune mechanics, diameter, morphology, and functionality like adhesion and flame retardancy. The fibers are passed through a first post-processing stage which has low temperature with low viscosity. In the second step, the fibers are faced by higher temperature and higher viscosity to manipulate desired functionalities.
For more information, click here.
Controlled Drug Delivery Systems

Our research team is working on core-shell nanofibers with different morphologies. Since the produced nanofibers are double-layered and the polymer shell layer is modified as porous and non-porous, the delivery of the drug, bioactive agents, additives, protein, growth factor, and etc. can be controlled over a prolonged period of time. Various studies have been carried out by our research group in medical applications where burst or prolonged drug release is necessary from antibacterial to wound healing, prolonged insulin release for diabetic patients avoiding frequently insulin intake and blood glucose measurement; the control and prevention of periodontal defects with controlled release.
For more information, click here


_edited.png)

